Paralyzed man walks after surgeons connect brain and damaged spine with Bluetooth
- Thread starter versitile1
- Start date
A new take onthat was cool.
what happens if someone hacks his Bluetooth/wifi signal?
The Manchurian Candidate?
Man Paralyzed For 12 Years Walks Again Thanks To Brain, Spinal Cord Implants
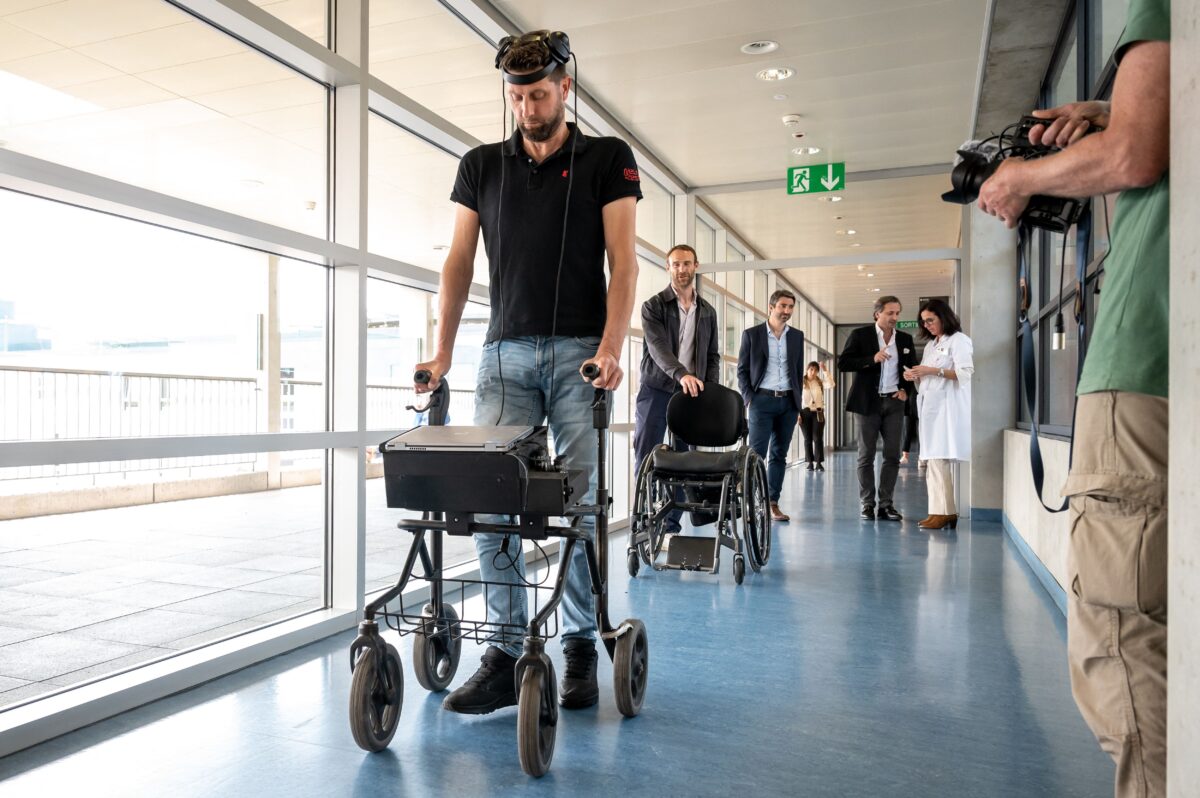
Man Paralyzed for 12 Years Walks Again Thanks to Brain, Spinal Cord Implants
A paralyzed man has been able to walk again for the first time in years simply by using the power ...
THURSDAY, MAY 25, 2023 - 06:20 PM
Authored by Katabella Roberts via The Epoch Times
A paralyzed man has been able to walk again for the first time in years simply by using the power of his mind thanks to implants fitted in his brain and spinal cord.
Gert-Jan Oskam, a 40-year-old Dutchman, was paralyzed in his legs and partially paralyzed in his arms following a cycling accident 12 years ago during which he suffered spinal cord damage.
He was told he would never walk again.
However, after being fitted with a device called a “brain–spine interface,” Oskam regained the ability to voluntarily move his legs and feet just by thinking about it, according to a study published May 24 in the journal Nature.
Walking naturally after spinal cord injury using a brain–spine interface - Nature
A reliable digital bridge restored communication between the brain and spinal cord and enabled natural walking in a participant with spinal cord injury.
“I feel like a toddler, learning to walk again,” Oskam told the BBC.

Brain implants help paralysed man to walk again
A paralysed man has been able to walk simply by thinking about it, thanks to electronic brain implants
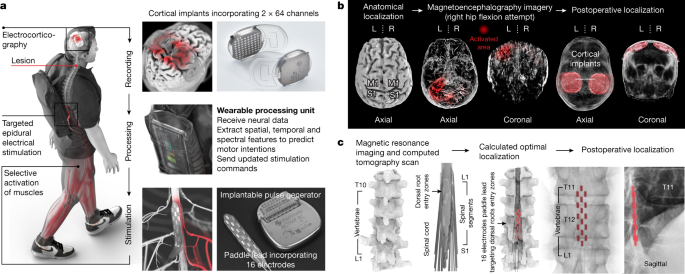
An international team of researchers, led by Dr. Grégoire Courtine, Professor Jocelyne Bloch, and others from the Swiss Federal Institute of Technology in Lausanne, fitted Oskam with the brain–spine interface, which works by creating a direct link between “cortical signals and the analogue modulation of epidural electrical stimulation targeting the spinal cord regions involved in the production of walking,” according to researchers.
How the Device Works
Put simply, the device restored the neurological link between the brain and the spinal cord, which is typically severed during accidents such as Oskam’s.
The device was implanted into Oskam’s skull, meaning it is not visible to the naked eye. When Oskam thinks about walking, the implant detects electrical activity in the cortex, the outer layer of the brain, and sends brain waves wirelessly to a computer that Oskam wears in a backpack.
The information is then transmitted to a pulse generator inserted into his spinal cord, effectively switching on muscles and allowing him to produce specific movements.

Gert-Jan Oskam, 40, victim of a spinal cord injury that left him paralyzed, walks with his implants during a press conference in Lausanne, Switzerland, on May 23, 2023. (Fabrice Coffrini/AFP via Getty Images)
Oskam also underwent around 40 rehabilitation sessions using the brain–spine interface, after which he regained the ability to voluntarily move his legs and feet.
Researchers believe Oskam’s movements would not have been possible with spinal stimulation alone and that the training sessions “prompted further recovery in nerve cells” which were not completely severed during his injury.
As well as being able to walk while using the device, Oskam can also walk short distances without the device, provided he uses crutches.
Read more here...
Wouldn't it be great if the hacker incorporated Michael Jackson 's "Beat it" ?that was cool.
what happens if someone hacks his Bluetooth/wifi signal?
Elon Musk owns a company involved in this research
Neuralink, Elon Musk's brain implant company, says it's received FDA approval for human trials

Brain implant company Neuralink says it's gotten permission from U.S. regulators to begin testing its device in people. The company is led by Elon Musk, whose silhouette is shown here behind the company's logo. (Dado Ruvic/Reuters)
Elon Musk's brain implant company, Neuralink, says it's gotten permission from U.S. regulators to begin testing its device in people.
The company made the announcement on Twitter Thursday evening but has provided no details about a potential study, which was not listed on the U.S. government database of clinical trials.
Officials with the Food and Drug Administration (FDA) wouldn't confirm or deny whether it had granted the approval, but press officer Carly Kempler said in an email that the agency "acknowledges and understands" that Musk's company made the announcement.
Neuralink is one of many groups working on linking the nervous system to computers, efforts aimed at helping treat brain disorders, overcoming brain injuries and other applications.
Earlier this week, for example, researchers in Switzerland published research in the journal Nature describing an implant that restores communication between the brain and spinal cord to help a man with paralysis to stand and walk naturally. There are more than 30 brain or spine computer interface trials underway, according to clinicaltrials.gov.

Musk, founder, CEO and chief engineer/designer of SpaceX, speaks during a news conference on Jan. 19, 2020, in Cape Canaveral, Fla. Musk's brain implant company, Neuralink, wants to one day implant computer chips inside people's brains. (John Raoux/The Associated Press)
Neuralink considered an 'investigational device'
Musk — who also owns Twitter and is the CEO of Tesla and SpaceX — said last December that his team was in the process of asking regulators to allow them to test the Neuralink device.
The device is about the size of a large coin and is designed to be implanted in the skull, with ultra-thin wires going directly into the brain.
Musk has said the first two applications in people would be to attempt to restore vision and try to help people with little or no ability to operate their muscles rapidly use digital devices. He also said he envisions that signals from the brain could be bridged to Neuralink devices in the spinal cord for someone with a broken neck.
After Musk made a presentation late last year about the device, Rajesh Rao, co-director of the Center for Neurotechnology at the University of Washington, said he doesn't think Neuralink is ahead of other teams in terms of brain-computer interface achievements, but is "quite ahead" in terms of the hardware in the devices.
It's unclear how well this device or similar interfaces will ultimately work, or how safe they might be. Neuralink's interface is considered an "investigational device" at this point, and clinical trials are designed to collect data on safety and effectiveness.
In its tweet this week, Neuralink said that it is not yet recruiting participants for the study and will provide more information soon.
Neuralink, Elon Musk's brain implant company, says it's received FDA approval for human trials
Brain implant company Neuralink says it's gotten permission from U.S. regulators to begin testing its device in people. The company is led by Elon Musk, whose silhouette is shown here behind the company's logo. (Dado Ruvic/Reuters)
Elon Musk's brain implant company, Neuralink, says it's gotten permission from U.S. regulators to begin testing its device in people.
The company made the announcement on Twitter Thursday evening but has provided no details about a potential study, which was not listed on the U.S. government database of clinical trials.
Officials with the Food and Drug Administration (FDA) wouldn't confirm or deny whether it had granted the approval, but press officer Carly Kempler said in an email that the agency "acknowledges and understands" that Musk's company made the announcement.
Neuralink is one of many groups working on linking the nervous system to computers, efforts aimed at helping treat brain disorders, overcoming brain injuries and other applications.
Earlier this week, for example, researchers in Switzerland published research in the journal Nature describing an implant that restores communication between the brain and spinal cord to help a man with paralysis to stand and walk naturally. There are more than 30 brain or spine computer interface trials underway, according to clinicaltrials.gov.

Musk, founder, CEO and chief engineer/designer of SpaceX, speaks during a news conference on Jan. 19, 2020, in Cape Canaveral, Fla. Musk's brain implant company, Neuralink, wants to one day implant computer chips inside people's brains. (John Raoux/The Associated Press)
Neuralink considered an 'investigational device'
Musk — who also owns Twitter and is the CEO of Tesla and SpaceX — said last December that his team was in the process of asking regulators to allow them to test the Neuralink device.
The device is about the size of a large coin and is designed to be implanted in the skull, with ultra-thin wires going directly into the brain.
Musk has said the first two applications in people would be to attempt to restore vision and try to help people with little or no ability to operate their muscles rapidly use digital devices. He also said he envisions that signals from the brain could be bridged to Neuralink devices in the spinal cord for someone with a broken neck.
After Musk made a presentation late last year about the device, Rajesh Rao, co-director of the Center for Neurotechnology at the University of Washington, said he doesn't think Neuralink is ahead of other teams in terms of brain-computer interface achievements, but is "quite ahead" in terms of the hardware in the devices.
It's unclear how well this device or similar interfaces will ultimately work, or how safe they might be. Neuralink's interface is considered an "investigational device" at this point, and clinical trials are designed to collect data on safety and effectiveness.
In its tweet this week, Neuralink said that it is not yet recruiting participants for the study and will provide more information soon.






